Limitations of non-invasive prenatal tests (NIPT)
In the case of structural chromosomal changes, fetal and maternal mosaics, in case of a polyploidy, of a vanishing twin, a fetoplacental discrepancy or maternal gonosomal aneuploidy
Due to the examination method non-invasive prenatal testing has certain limits. Therefore please inform your patients about these limitations as follows:
Structural chromosomal changes, mosaics and polyploidy
In general, no statements regarding structural chromosomal changes, mosaics or polyploidy can be made with the PrenaTest®.
Fetal mosaics, fetoplacental discrepancies
The examined fetal DNA is primarily derived from the cytotrophoblast and is released by apoptosis and necrosis of trophoblast cells of the placenta. As such, it is only possible to achieve a level of diagnostic certainty close to that attained via direct chorionic villus sampling. Consequently, mosaics or fetoplacental discrepancies in trisomies 21, 18 or 13 resp. gonosomal aneuploidy are not recognisable. In the event of a fetoplacental discrepancy, this can also mean that the PrenaTest® result is not representative for the unborn child.
Vanishing Twin
Undisclosed vanished twins can contribute a sufficient proportion to the total cffDNA fraction to cause a positive PrenaTest® result being not representative for the continuing singleton pregnancy.
Maternal mosaic
An existing maternal mosaic can lead to a conspicuous PrenaTest® result which may not representative for the unborn child. These mosaics primarily affect gonosomal aneuploidy. For example, cases of pregnancies in women affected with Turner syndrome were based on mosaic findings (45, X/46, XX) in the women.
Maternal gonosomal aneuploidy
An existing maternal gonosomal aneuploidy as the Triple X syndrome can lead to a conspicuous PrenaTest® result which may not be representative for the unborn child. This means, for example, that a positive PrenaTest® result with a reference to a chromosome disorder 47, XXY does not necessarily represent a fetal chromosomal abnormality. Rather, it should be examined whether and how the pregnant woman has a triple X syndrome.
Other maternal genetic disorders
Maternal tumors, copy number variants (CNV), as well as rare genetic modifications in the examined gene regions, can very rarely lead to a discordant PrenaTest® result, which is not representative for the unborn child.
Mother is carrier of the 22q11.2 microdeletion
It is possible that the mother is the carrier of the 22q11.2 microdeletion, associated with the DiGeorge syndrome and velo-cardio-facial syndrome (Shpintzen syndrome) but not the unborn child. This can lead to discordant (false-positive) test results
Size of the microdeletion
In more than 85% of the affected persons, the deletion includes a region measuring approx. 2.5 megabases in the 22q11.2 region of chromosome 22. This region is investigated with the PrenaTest®. A small percentage of affected persons has an even smaller deletion or point mutation in the affected region which cannot be detected with the PrenaTest®. This can lead to discordant (false-negative)
test results.
Please note – a predictive value of 100% cannot be expected
LifeCodexx work is state of the art in terms of science and technology. LifeCodexx notes that a validity of 100% in the use of the PrenaTest® at the practice cannot be expected. There are risks that can never be fully excluded, no matter how meticulous the genetic analysis is. Nevertheless, all possible measures and safety precautions are undertaken to avoid these risks and other errors.
Fetoplacental discrepancies
Mosaics limited to the placenta associated with intrauterine growth disorders
Depending on when and in which stage of embryonic development faulty chromosomal division occurs, different tissues (fetal and/or extra-fetal) are affected by it. Fig. 1a shows an example of a mosaic limited to the placenta which can lead to a false-positive or, rather, a discordant test result: here, the fetus is not affected by the chromosomal disorder and invasive diagnostic procedures would reveal an unremarkable karyogram. However, mosaics limited to the placenta can also be medically relevant, since they may be associated with intrauterine growth disorders1. Fig. 1b shows the mosaic distribution of a chromosomal disorder which only affects the fetus, however not the placenta. In such a case, the test result would probably be false-negative. There are still few data about the frequency with which such fetoplacental discrepancies occur. Wegner and Stumm report that in 1–2 % of chorionic villi examined, mosaics limited to the placenta were able to be detected2 Despite a high degree of accuracy of NIPT, there will always be discordant test results. While invasive diagnostic procedures can, as a general rule, be avoided in the case of an unremarkable test result – in the context of all relevant clinical findings and after appropriate explanation provided to the pregnant woman – positive test results must be diagnostically clarified using invasive methods, according to the recommendations of professional associations.
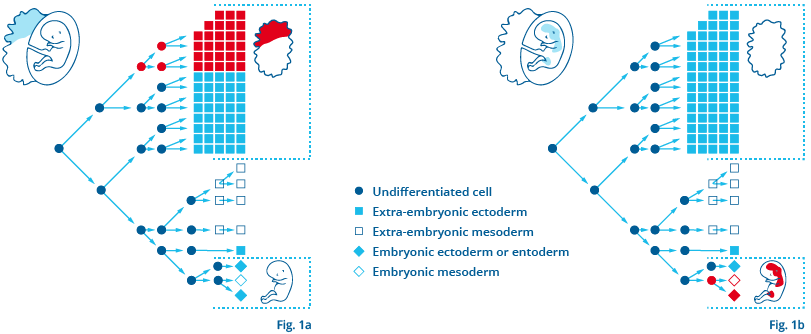
Left: Fig. 1a / Right: Fig. 1b
