PrenaTest® Performance Qualification
PrenaTest® for the determination of fetal trisomy 21 (test option 1)
The performance of the PrenaTest® Option 1 has been validated 2019 in a clinical study. The study results of the maternal plasma samples (n=1062) demonstrated a positive percentage agreement (PPA; equates to sensitivity) of 99,9 (lower 1-sided 95% confidence interval of 91.8 %; n=42/42) and a negative percentage agreement (NPA; equates to specificity; n=1019/1020) of 99,9 compared to NGS-based PrenaTest®. The negative predictive value (NPV) was 100 % (lower 1-sided 95 % confidence interval of 99.71%). The average fetal fraction of all examined blood samples was 10.55%. The qNIPT delivered also reliable results in a previous study at 54 blood samples with a fetal fraction below 4% and as low as 2.4%. The PrenaTest® Option 1 can be applied in the case of singleton pregnancies.
PrenaTest® Option 2 and 2 Plus as well as Option 3 and 3 Plus
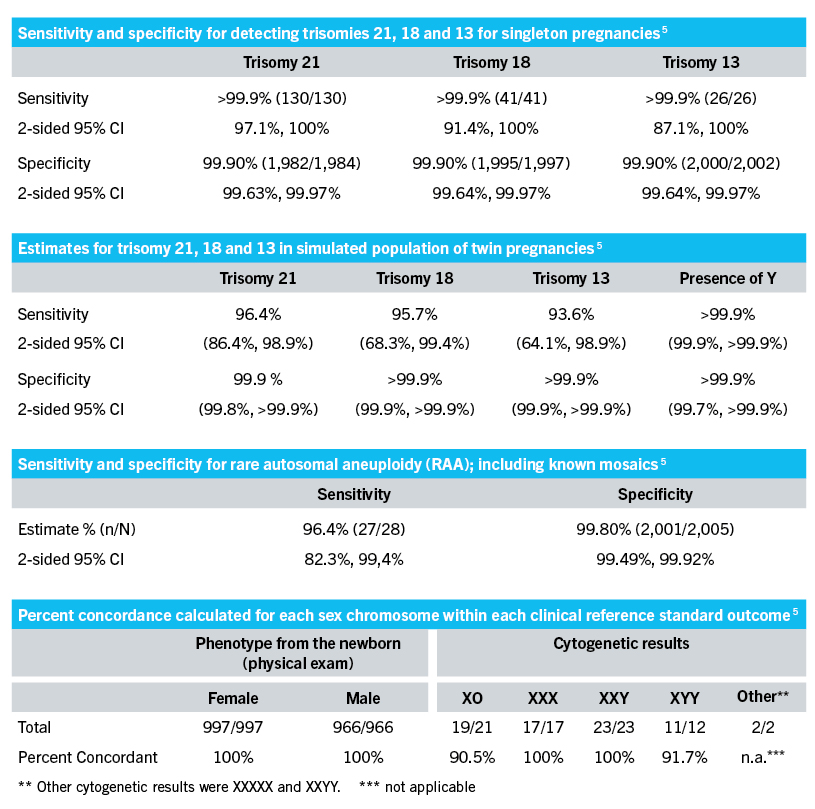
Source: VeriSeq NIPT Solution v2 Package-Insert_Dok-Nr. 1000000078751 v06_Aug2021
PrenaTest® for the detection of the 22q11.2 microdeletion (optional for test options 2 oder 3)
Phase 1
A total of 469 samples were tested, of which 175 (37.3%) met the quality criteria. Three positive samples with a 22q11.2 microdeletion were correctly determined (3/3, 100%). All negative samples were correctly classified (172/172, 100%). There were no false-positive or false-negative results.
Phase 2
In a final internal blinded study, 20 samples from Phase 1 were examined. All samples were classified correctly. Due to the low number of cases a concrete test sensitivity and specificity cannot be derived.
Fetal gender determination
For PrenaTest® Option 1 the determination of the fetal gender is based on the proprietary qPCR assay QuantYfeX®. The assay applied for fetal gender determination has not been validated in a clinical study; however, it has successfully completed an internal methodological-technical validation, in which 1160 samples were examined. A –male– result is reported, if a Y chromosomal marker (SRY) is detected by QuantYfeX®. A –female– result is reported, if a Y chromosomal marker (SRY) is not detected by QuantYfeX®. In very rare cases the fetal gender cannot be determined. In this case the fetal gender is not definable and the analysis will not be repeated.
Literature
Stumm et al. 2012. Noninvasive prenatal detection of chromosomal aneuploidies using different next generation sequencing strategies and algorithms. Prenatal Diagnosis 2012, 32, 569–577
Stumm et al. 2013. Diagnostic accuracy of random massively parallel sequencing for non-invasive prenatal detection of common autosomal aneuploidies: a collaborative study in Europe.Prenatal Diagnosis 2013, 33,1 –7
Groemminger et al. 2014. Fetal Aneuploidy Detection by Cell-Free DNA Sequencing for Multiple Pregnancies and Quality Issues with Vanishing Twins. J. Clin. Med. 2014, 3, 679-692; doi:10.3390/jcm303067
Floeck et al. 2017. Non-invasive prenatal testing (NIPT): Europe’s first multicenter post-market clinical follow-up study validating the quality in clinical routine. Arch Gynecol Obstet DOI 10.1007/s00404-017-4517-3
Hofmann et al. 2014. NIPT: Welche Unterschiede zwischen den Tests gibt es tatsächlich? FRAUENARZT 55 (2014) Nr. 11
Groemminger et al. 2015. The influence of low molecular weight heparin medication on plasma DNA in pregnant women. Prenatal Diagnosis 2015, 35, 1–3
Wolf et al. 2016. Purification of Circulating Cell-Free DNA from Plasma and Urine Using the Automated Large-Volume Extraction on the QIAsymphony® SP Instrument. © Springer International Publishing Switzerland 2016 P.B. Gahan et al. (eds.), Circulating Nucleic Acids in Serum and Plasma – CNAPS IX, Advances in Experimental Medicine and Biology 924, DOI 10.1007/978-3-319-42044-8_33